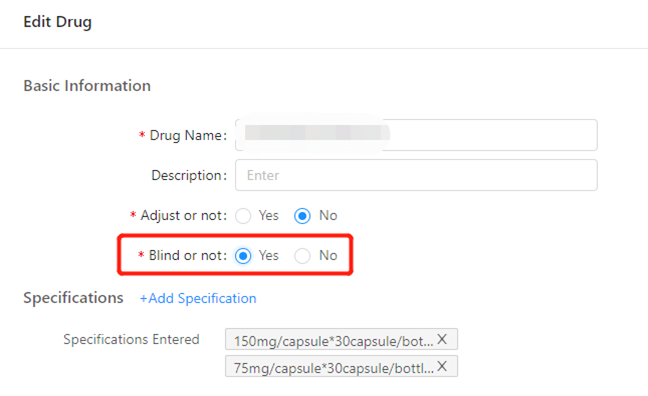
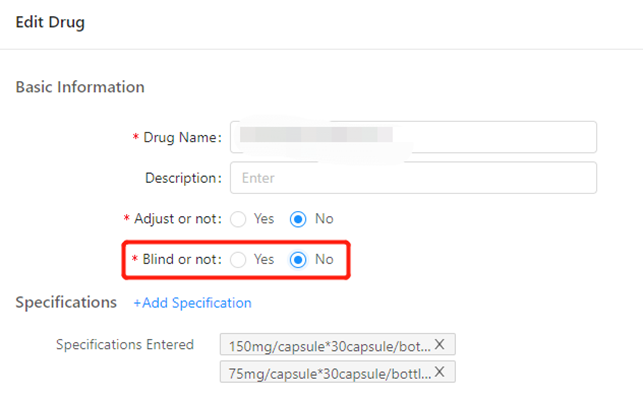
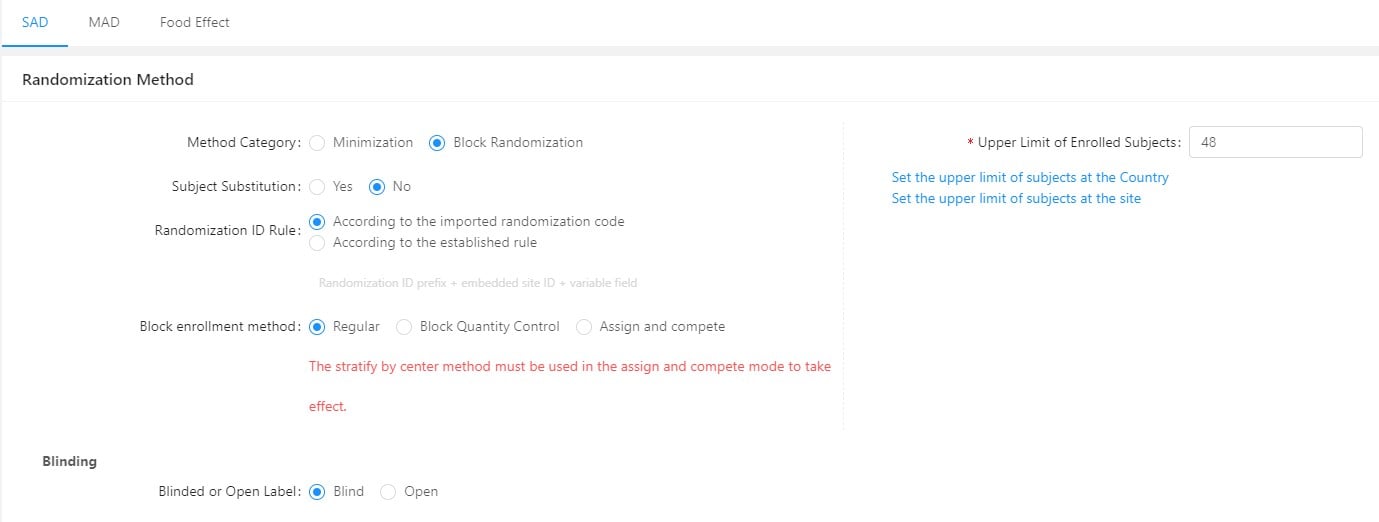
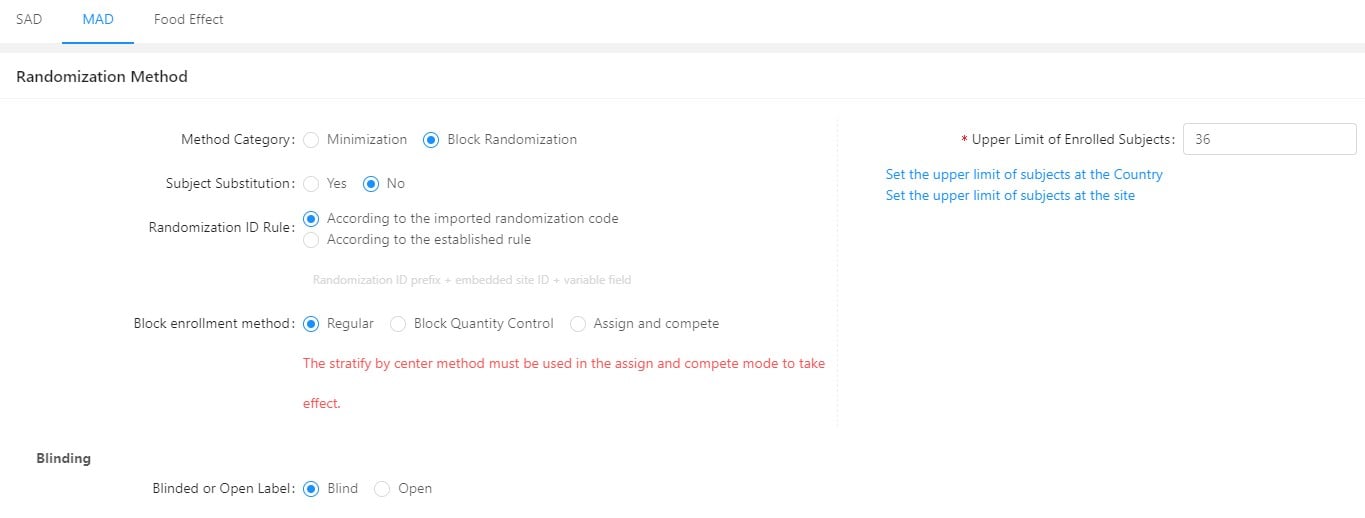
GreenLight Clinical and Taimei Technology partner to accelerate clinical research
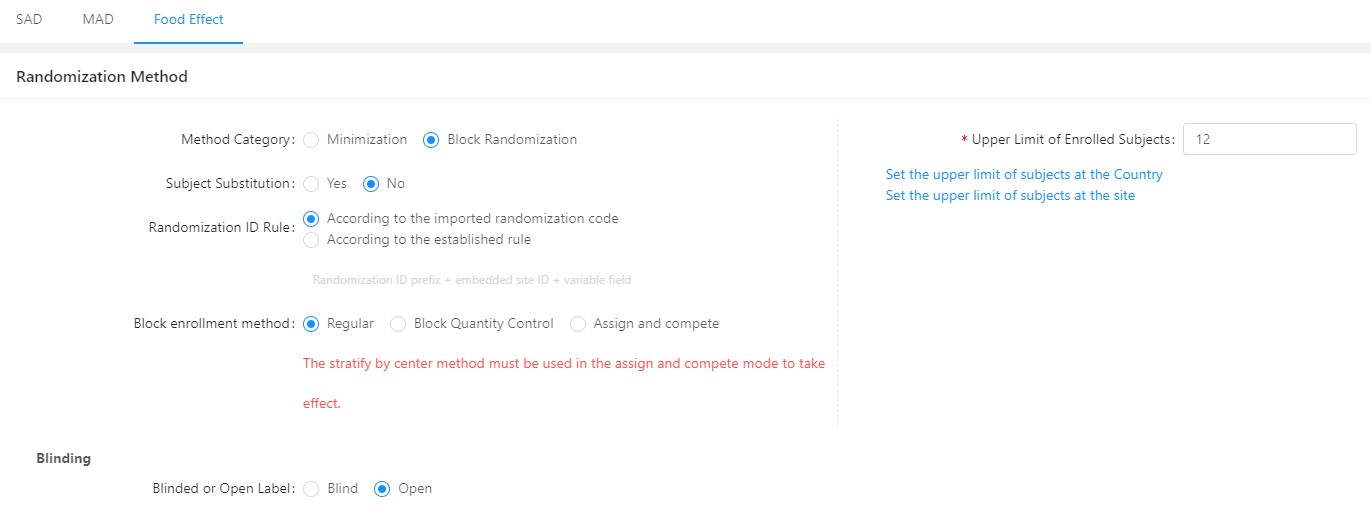
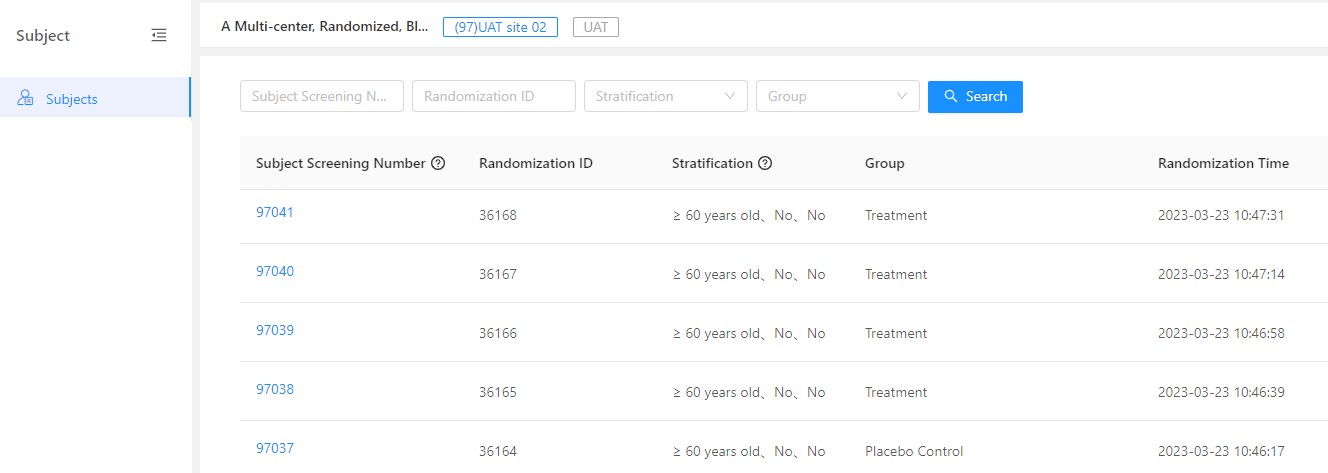
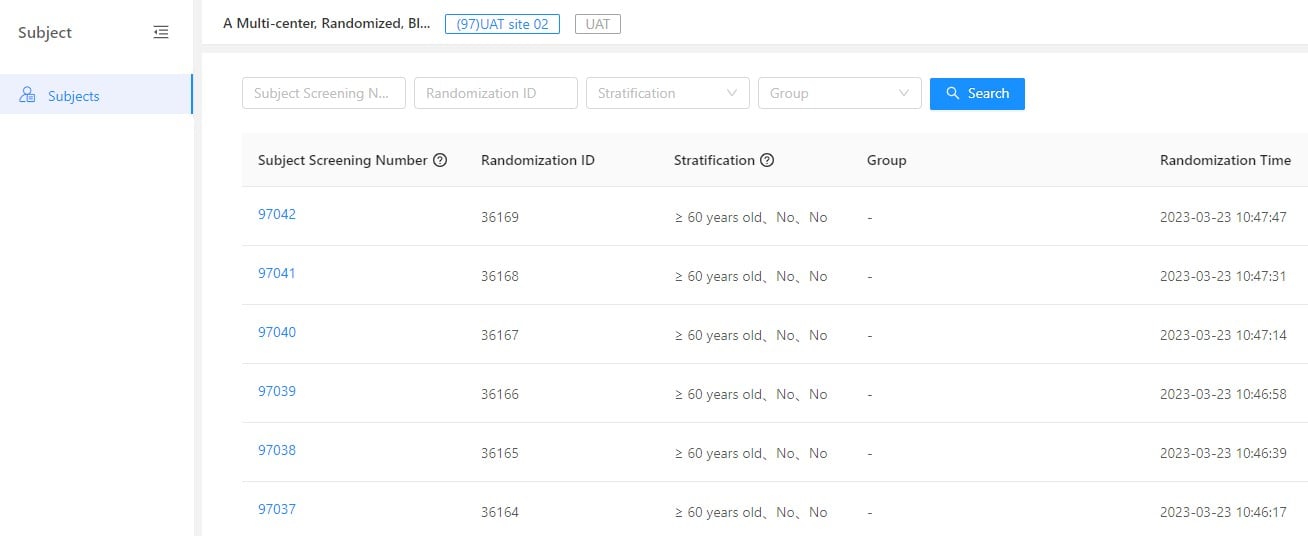
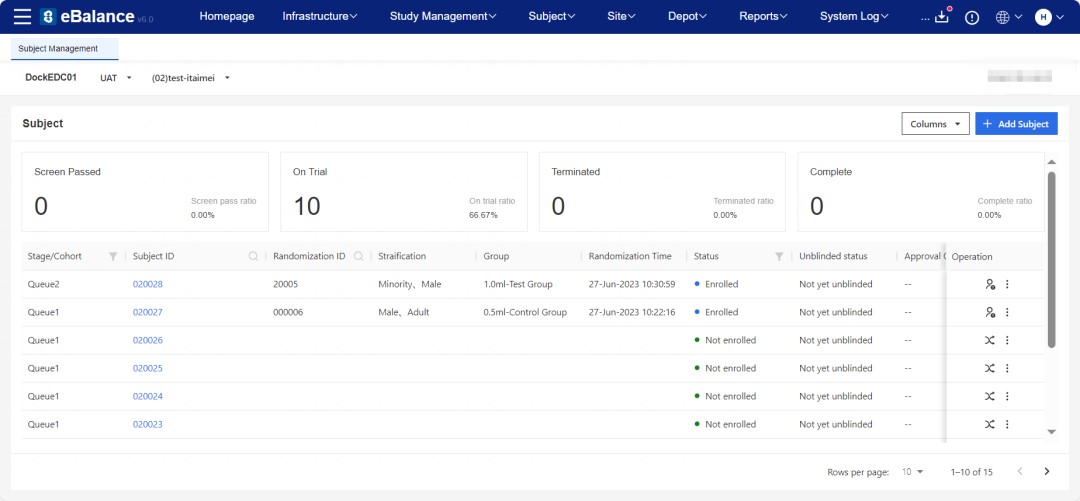
Reading Time: 3 minutes GreenLight Clinical, a physician-led solutions-focused Clinical CRO, and Taimei Technology have joined forces to accelerate clinical research. This partnership leverages Taimei’s AI-driven