
End-to-End Quality and Compliance in Drug Safety Monitoring

Comprehensive Pharmacovigilance
throughout the Entire Product Lifecycle

Pre-Market + Post-Market
Product Safety Data

GVP & Regulatory Compliance and Efficient Operations

GVP-Based Unified Management
Unified management of institutions, personnel, policies, and resources based on GVP requirements
Digital System Visualization
Visualize system management and pharmacovigilance activities

Real-Time Data Synchronization
Generate and automatically update the main pharmacovigilance system files with one click

Automated Compliance Checks
Automatically detect compliance issues and provide intelligent corrective guidance
Collect, process, and submit safety data from various sources with risk control and management
Unified management of adverse reaction reports with statistical analysis, signal alerts, and audit trails
Integration with CDE and CDR systems for direct adverse reaction reporting via E2B
Compliant with global pharmacovigilance databases, supporting direct submissions to FDA FAERS and EU EudraVigilance
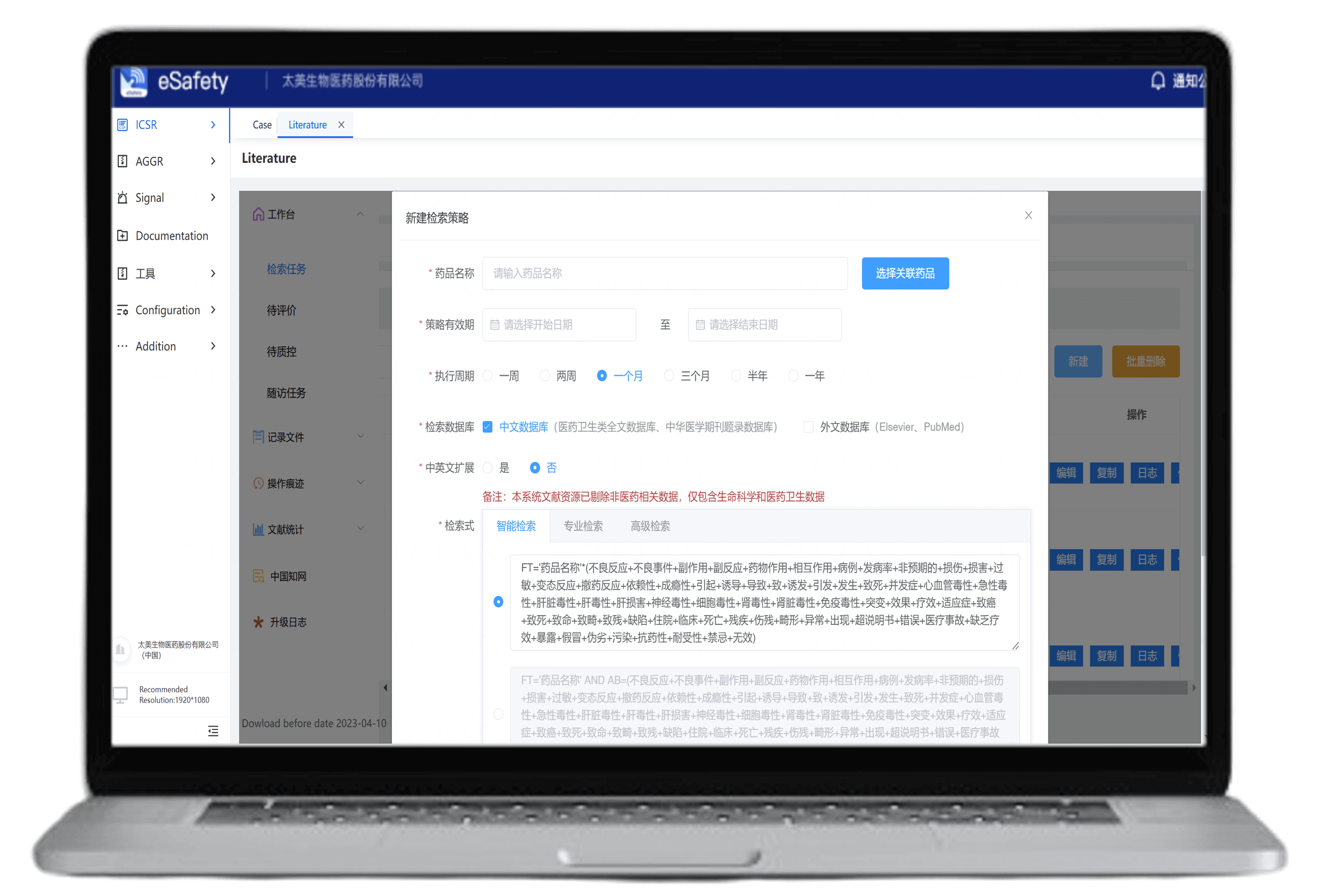
Supports searches across CNKI, Chinese Medical Journals, PubMed, and Elsevier in both English and Chinese
Ensures compliance with search sources
Customizable search queries and frequency settings
Compliant full-text downloads with search traceability

Automatically collect AE data from FDA, WHO, EMA, and eSafety databases. Invalid reports are filtered out, with multi-dimensional views of product overviews, report distribution, signal lists, and trends

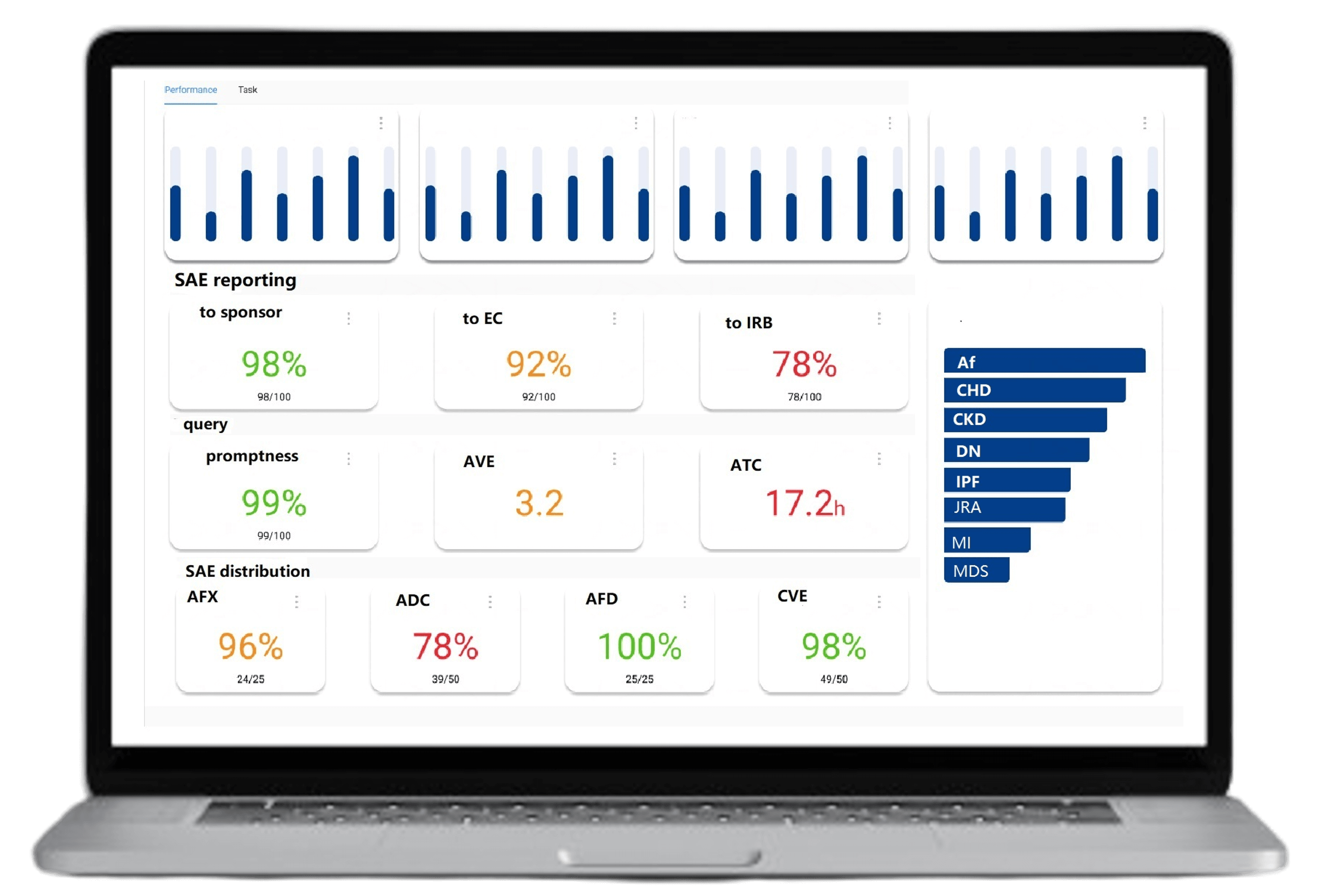
Over 35 statistical charts updated daily, providing in-depth analysis of individual case reports. Exportable in Excel, PDF, and other formats for ease of use
Visualize risks and quickly identify pharmacovigilance compliance issues. Automatically generate risk lists, solutions, and risk review reports
Using humanoid robots for pharmacovigilance data processing, including MedDRA coding, document archiving, and individual case report tracking
Automating repetitive, mechanical, and rule-based tasks reduces labor costs and error rates while enhancing work efficiency

Scan to Follow Us on LinkedIn

Scan to Follow Us on WeChat
